Warning: Directory /usr/share/nginx/html/en/data/cache/URI not writable, please chmod to 777 in /usr/share/nginx/html/en/plugin/htmlpurifier/HTMLPurifier.standalone.php on line 15750
Warning: Directory /usr/share/nginx/html/en/data/cache/URI not writable, please chmod to 777 in /usr/share/nginx/html/en/plugin/htmlpurifier/HTMLPurifier.standalone.php on line 15750
Warning: Directory /usr/share/nginx/html/en/data/cache/URI not writable, please chmod to 777 in /usr/share/nginx/html/en/plugin/htmlpurifier/HTMLPurifier.standalone.php on line 15750
[Linked in] Mar 3rd K-BPR : Guidance of approval for biocidal substances and products
Hello, we are back!
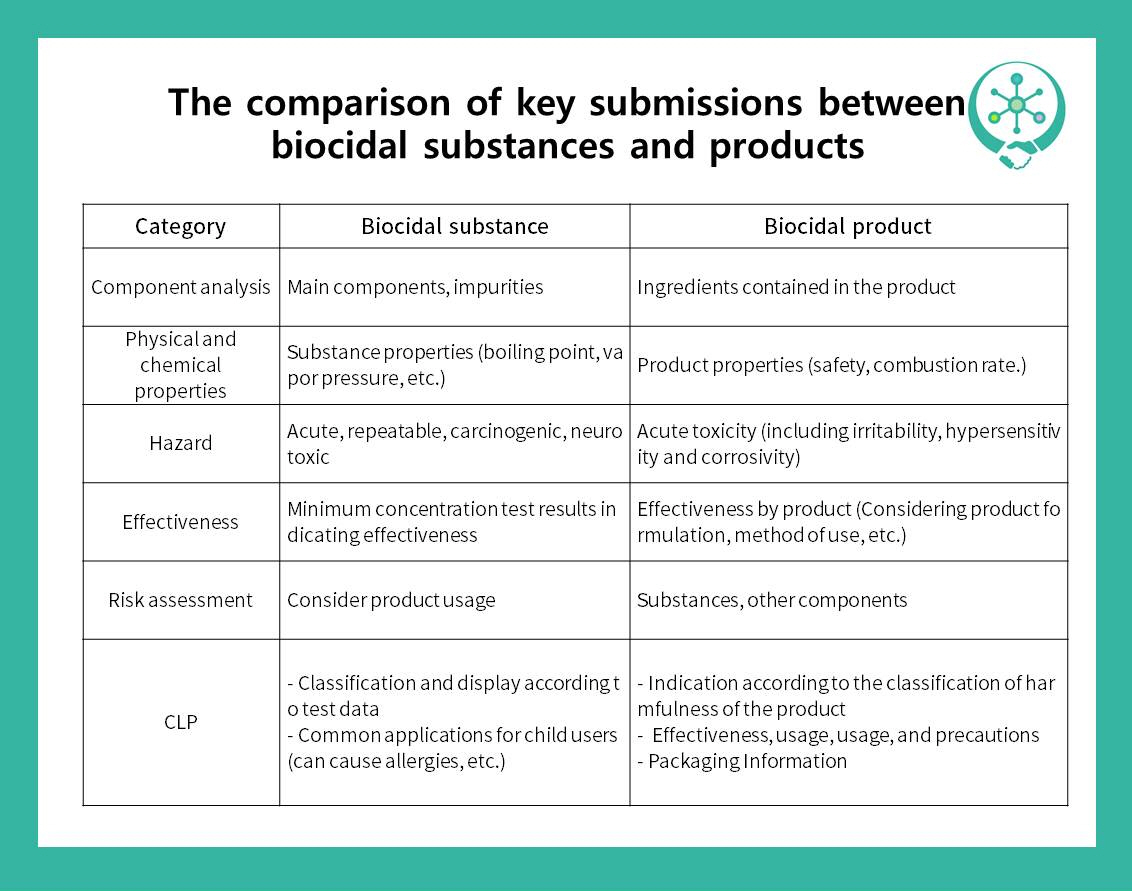
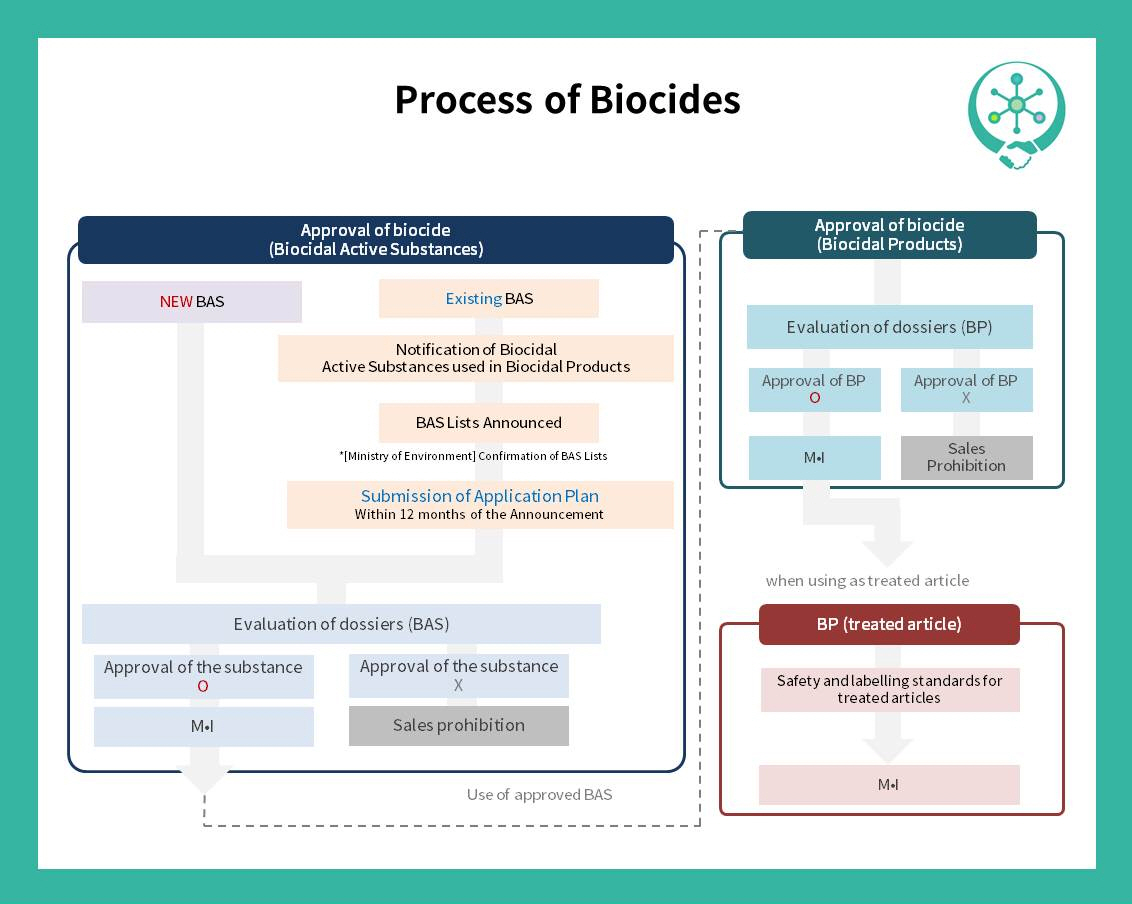
For today, we would like to share the guidance of approval for biocidal substances and products. The person who wants to manufacture or import the biocidal substances for producing biocidal products should submit the document to the National Institute of Environmental Research and will receive the Substance Authorization Initiation Notice.
Then, he/she have to submit the draft evaluation sheet within 1 year, and the substance will be approved after the evaluation in 30days. The case of the biocidal products is similar to the substance, but the difference is they will receive the Product Authorization Initiation Notice and should submit the evaluation document in 6 months. Approval after the evaluation would be in 30days as well. Check out the cards for details :D
For the next week will be the Approval criteria for biocidal substances and products. Any inquiries related to K-BPR, please feel free to contact info@safety-as.com or +82-27540600. Have a great day!
▶ Join our group and get the recently news!: https://www.linkedin.com/groups/13911952/