The purpose of K-REACH is to protect public health and the environment from
the hazards and risks of chemicals.
K-REACH enforced in 2015, those who manufacture or import chemicals (more than 1 t/y of
phase-in substances,
and more than 0.1 t/y of non-phase-in substances) are obligated to
register chemicals in accordance with the revised contents in 2019.
Notification subject: A person who intends to manufacture
or import less than 0.1 ton of non-phase-in substance
Registration subject : A person who intends to manufacture or
import more than one ton of phase-in substance and more than 0.1 ton of non-phase-in substance
1) Phase-in substances :
1. Chemical substances publicly notified by the Minister of Environment in consultation with the Minister of Employment and Labor, which were domestically distributed for commercial purposes before February 2, 1991
2. Chemical substances publicly notified by the Minister of Environment and where the hazard reviews thereof have been conducted pursuant to the former Toxic Chemicals Control Act after February 2, 1991
2) Non-phase-in substances : All substances except the phase-in substances
3) Substances subject to intensive control :
1. A substance that causes or is likely to cause cancer, mutation, reproductive disorders or disorders of the endocrine system in humans or animals
2. A substance that is highly likely to accumulate in human body animals or plants and remains in the environment for an extended period of time
3. A substance that, when exposed to humans, may cause damage to the internal organs such as lungs, liver, and kidneys
4. A substance that may pose a risk equivalent to or more serious than the substances referred to in items (1) through (3) to humans, animals or plants
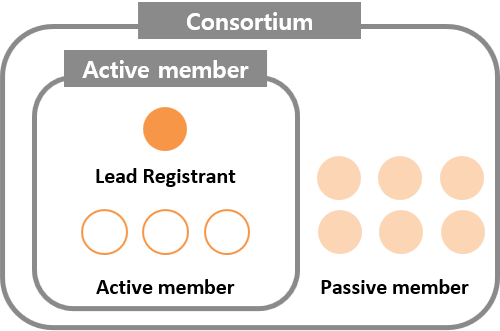
A person who intends to register phase-in substances within the registration grace period shall proceed with the joint registration by forming a consortium in accordance with Article 15 of K-REACH (Method of submitting materials when applying for the existing chemicals).
Organizing a consortium
1. Lead registrant : A person who represents the consortium in activities for joint registration of the phase-in substances
Active member : Those who are active in discussing and making decisions with the lead registrant in the activities for joint registration of the phase-in substances
3. Passive member : Those who purchase LoA to proceed with the registration according to the decisions made in the activities for joint registration of the phase-in substances
1. Organizing a consortium
• Joining a consortium, electing a lead registrant, gathering active members, and identifying the sameness, etc.
2. Consortium Agreement conclusion
• Discussion of consortium operation and cost-sharing method, etc.
• Conclusion of joint registration operation agreement
3. Data Purchase and production
• Data Gap Analysis, Checking member's own test data and LoA, Planning for purchase/generation of test data, and Purchase/generation of test data
4. Preparation of Registration dossier
• Preparation of Classification and labeling, risk data, and Registration dossier
5. Registration
• Submission of LR's registration dossier, LoA sale, and Preparation and submission of member's registration dossier